GCSE / KS4 Science Bundle - Save 10%!
Buy our GCSE/KS4 bundle and save 10%!
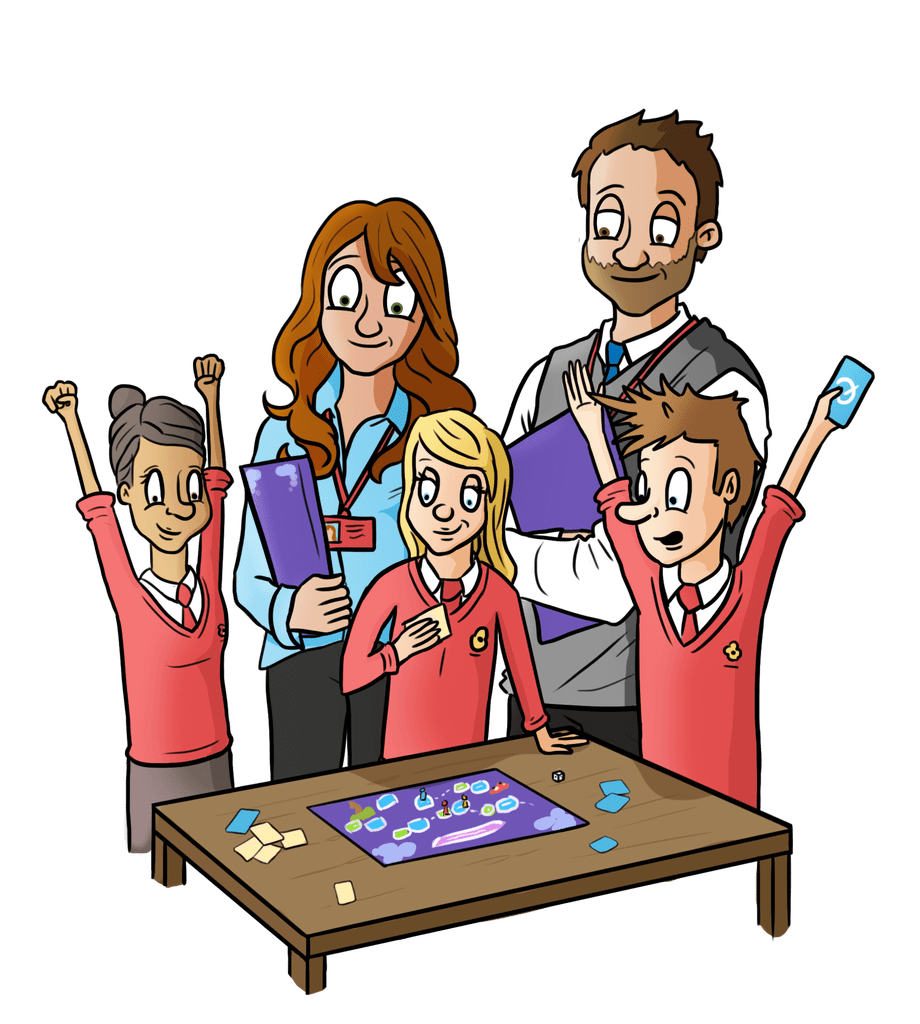
Revolutionise the way you learn with Oaka Digital
Designed for Dyslexics, Effective for Everyone
Which type of Oaka product do I need?
How Oaka works in the classroom...
Read what other teachers are saying!
Online learning the Oaka way!
👍 Suitable for AQA, OCR, Edexcel, WJEC, and CCEA exam boards
Buy our GCSE/KS4 bundle and save 10%!

Designed for Dyslexics, Effective for Everyone
✅ Learn or revise complicated concepts easily
✅ Information broken down into short chunks
✅ Full-colour illustrations on every page
Arguably the basis of chemical investigation, the Periodic Table has developed over many years. This Periodic Table revision pack will help students get to grips with the different elements, where they can be found in the table and why they are in those positions. This revision pack makes learning the core information quick and simple.
Broken down into bite-sized chunks, this Periodic Table GCSE revision guide will help students understand (and remember!) the key facts about the Periodic Table, its history and uses.
Each numbered section is clearly laid out and illustrated to aid understanding, making it more accessible to all students, irrespective of their reading ability, with beautiful images to highlight each key point.
Written by Dr Timothy Danks, former Head of Science at The Oratory School and a tutor for over 25 years, the Periodic Table revision pack brings GCSE revision to life in a visual and highly memorable way, helping students understand and apply this knowledge to their study of chemistry.
💡Plus, the Topic Pack contains 52 active learning questions & answer flashcards!
This is a complicated topic that is made clear and easy to understand in this fully illustrated topic pack.
The periodic table is a tabular arrangement of chemical elements organised on the basis of their atomic number, electron configurations and chemical properties. The elements are arranged in rows and columns according to their increasing atomic number, with elements having similar chemical properties being placed in the same vertical column, known as a group or family.
The elements in the same row, known as a period, have similar electron configurations. The table also includes information about the element’s symbol, atomic number, and atomic weight. The periodic table has been expanded numerous times since its inception in the 19th century when scientists first began to systematically study chemical elements. The earliest version of the table was created by the Russian chemist Dmitry Mendeleev in 1869. Mendeleev arranged the known elements in order of increasing atomic weight and noticed that certain elements shared similar chemical properties. He left gaps in his table where he predicted new elements would be discovered, and his predictions were duly confirmed by the discovery of those elements.
Since Mendeleev’s original table was created, scientists have continued to discover new elements and refine the table to better reflect the elements’ properties. The modern periodic table includes 118 elements, and scientists continue to explore the potential existence of additional elements beyond those currently known.
Learning about the periodic table for GCSE is very useful, not only because questions could be based around it but because it’s foundational to modern chemistry.
The periodic table has become an essential tool for organizing and understanding chemical elements, and it continues to play a critical role in many scientific disciplines. Periodic table GCSE learning materials are essential for GCSE/KS4 chemistry.
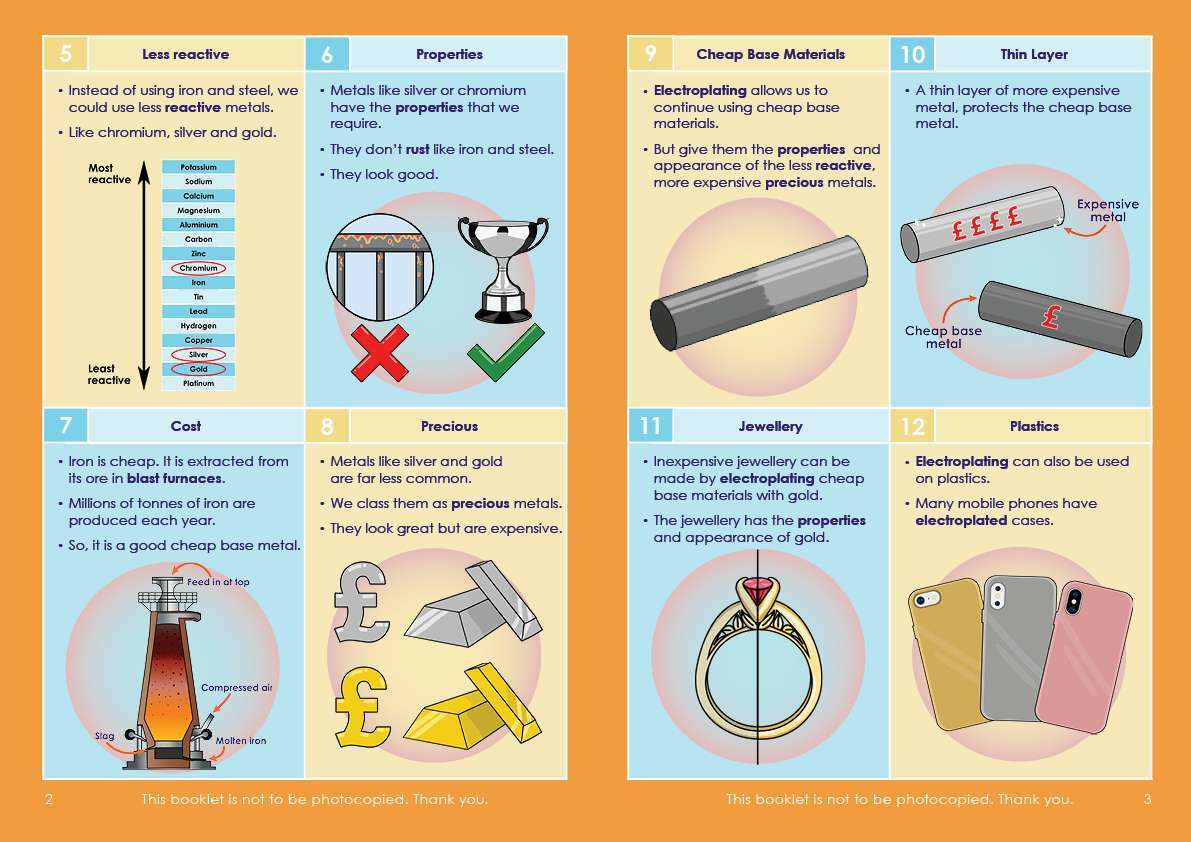
Engaging, full-colour illustrations on every page
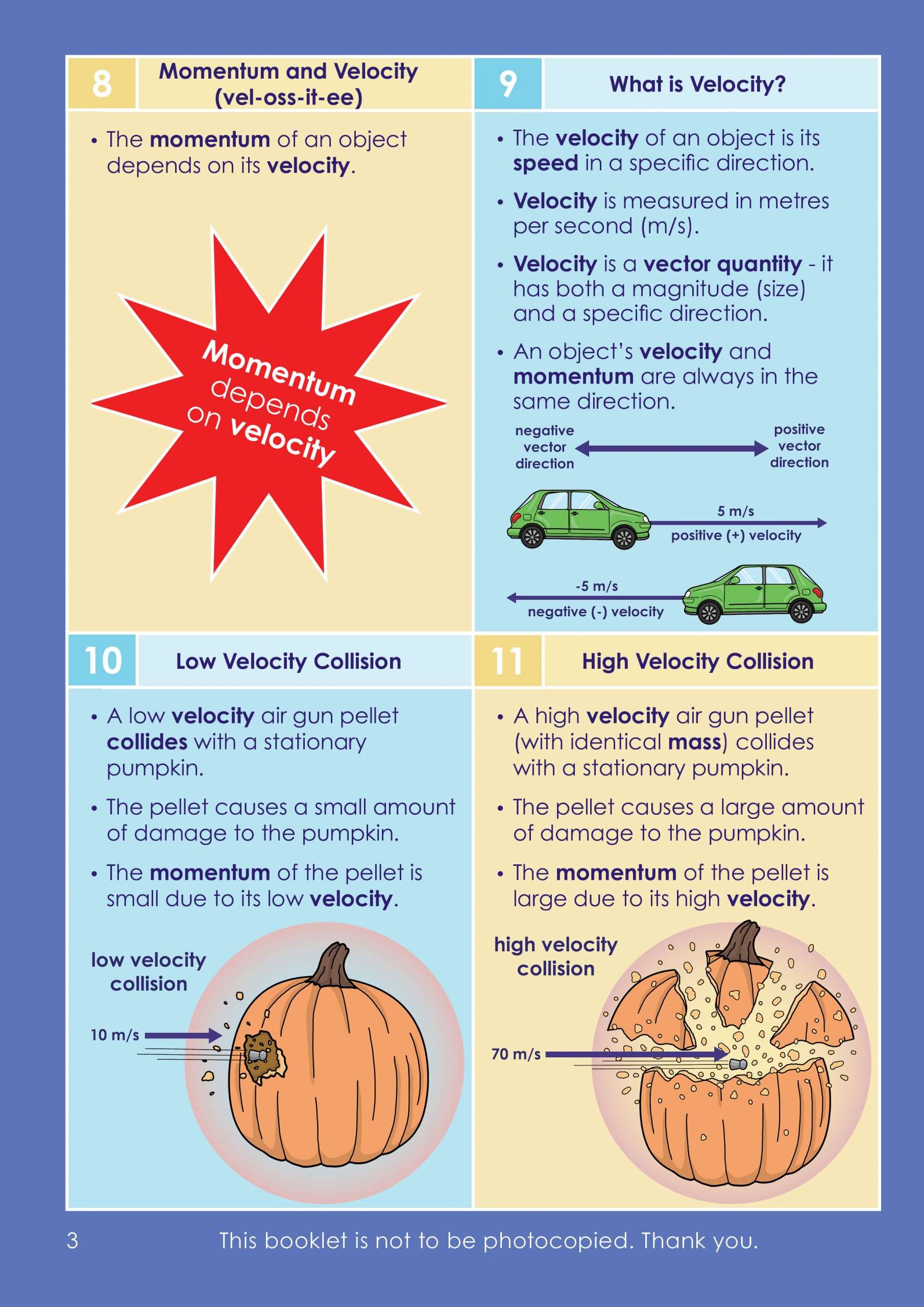
Text broken down into bite-sized chunks on a lightly shaded background
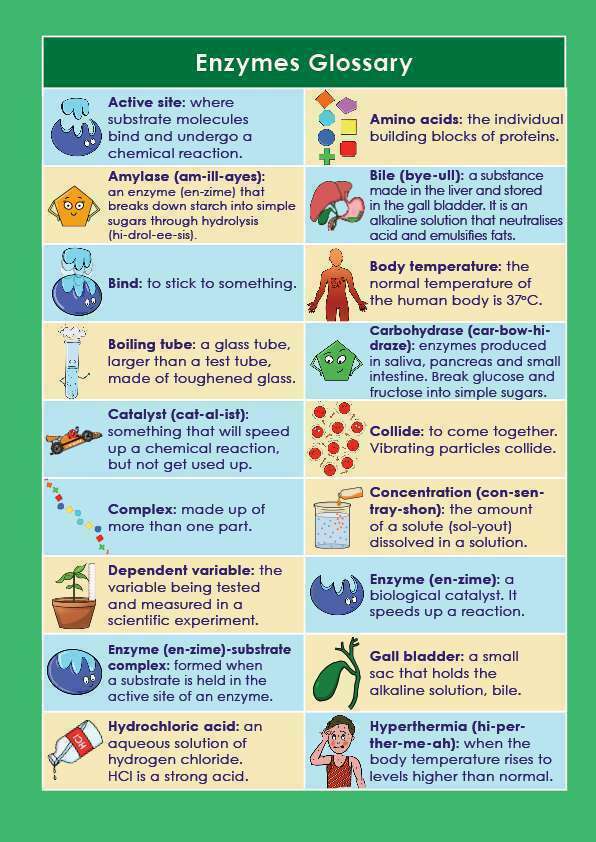
A simple, easy-to-understand glossary of key terms
Topic Booklet
Write Your Own Notes Booklet
Active Learning Q&A Flashcards
Topic Booklet: ✅ x1
Write Your Own Notes Booklet: ✅ x1
Active Learning Q&A Flashcards: ✅ x1
BEST VALUE!
Topic Booklet: ✅ x1
Write Your Own Notes Booklet: ❌
Active Learning Q&A Flashcards: ❌
Topic Booklet: ❌
Write Your Own Notes Booklet: ✅ x1
Active Learning Q&A Flashcards: ❌
Please note, our resources are NOT to be photocopied. Thank you.
The range of Oaka books and digital platform is absolutely fantastic at helping students with SEN needs to revise key topics in a way that helped hek to breakdown and remember key facts!!!
Great. Just what my son needed. Explains everything clearly, with simple pictures to help with recall. Flashcards were brilliant too. Simple, clear, no clutter, not busy. Helped my son get a really good grade in his test. I just wish you had the GCSE ones for every subject, as I would buy them.
Girls love it. Will hopefully make the topic more memorable.
Chemistry
Physics
Physics
Chemistry
