CE / KS3 Science Bundle - Save 10%!
Buy our CE/KS3 bundle and save 10%!
Revolutionise the way you learn with Oaka Digital
Designed for Dyslexics, Effective for Everyone
Which type of Oaka product do I need?
How Oaka works in the classroom...
Read what other teachers are saying!
Online learning the Oaka way!
Buy our CE/KS3 bundle and save 10%!

Designed for Dyslexics, Effective for Everyone
✅ Learn or revise complicated concepts easily
✅ Information broken down into short chunks
✅ Full-colour illustrations on every page
Acids are substances that release hydrogen ions when dissolved in water. Common examples of acids include lemon juice, vinegar and various acids.
Alkalis, sometimes referred to as bases, release hydroxide ions when dissolved in water. Common examples of alkalis include baking soda and lye.
An indicator is a substance that changes colour in the presence of an acid or an alkali. Indicators can be used to determine the pH of a solution, which is a measure of its acidity or basicity. Examples of indicators include litmus paper, red cabbage juice, and phenolphthalein.
The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline. A pH of 7 is neutral, meaning it is neither acidic nor alkaline. A pH less than 7 is acidic, and a pH greater than 7 is alkaline. Indicators are used to determine the pH of a solution by observing the colour change of the indicator in the solution.
Understanding the properties of acids and alkalis and how to use indicators to determine pH, is essential in many fields, such as chemistry, biology, and medicine. It is also very important in the lab or field of research, as well as in industry and everyday life, in order to ensure safety and the correct use of chemicals.
Substances that change colour when exposed to acids and alkalis are called indicators. Two common examples are litmus and red cabbage.
Litmus is a natural pH indicator made from lichens. It turns red in acidic solutions and blue in basic solutions.
Red cabbage indicator is a pH indicator made, as the name suggests, from red cabbage. It changes colour depending on the pH of the solution it is added to. Red cabbage indicators are red in acidic solutions, purple in neutral solutions, and blue in alkali solutions.
Our acids and alkalis KS3 learning materials cover all topics in detail with easy-to-digest information and plenty of visual learning aids.
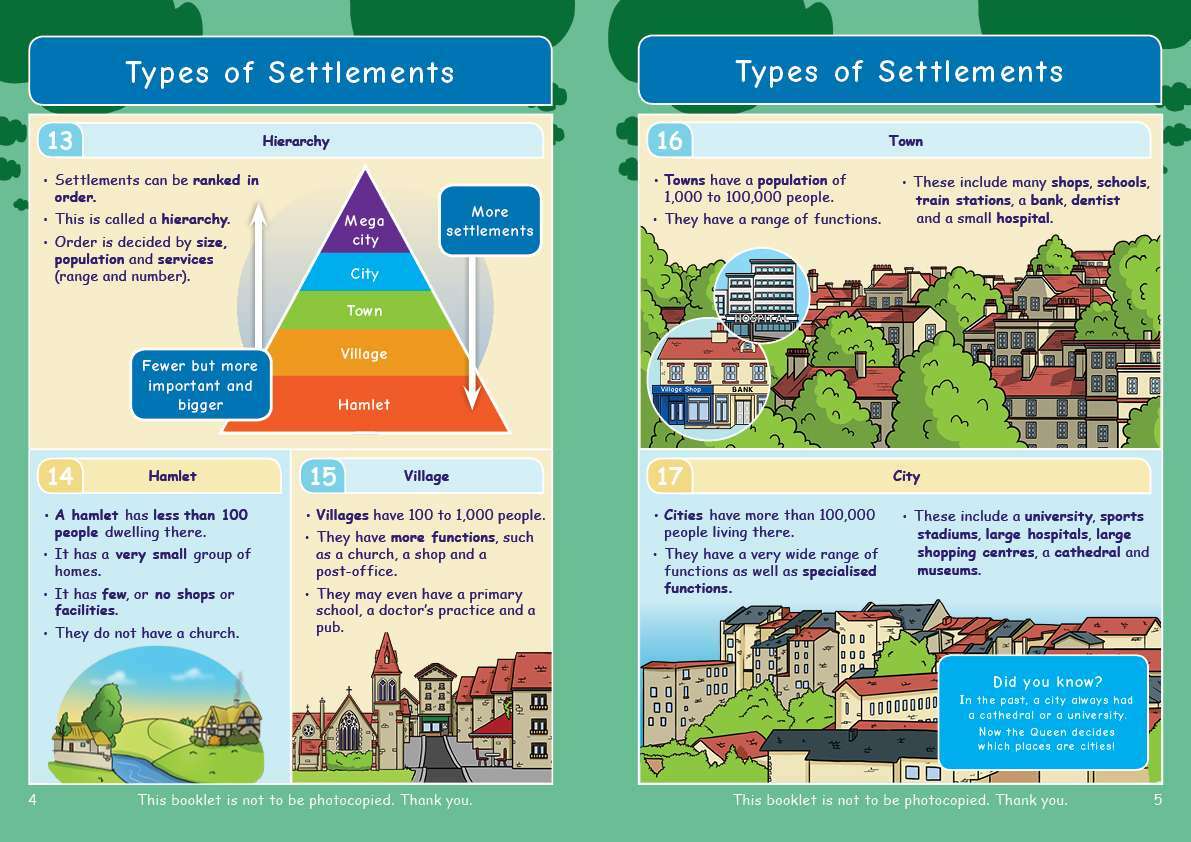
Engaging, full-colour illustrations on every page

Text broken down into bite-sized chunks on a lightly shaded background

A simple, easy-to-understand glossary of key terms
Topic Booklet
Write Your Own Notes Booklet
Active Learning Game or Map
Topic Booklet: ✅ x1
Write Your Own Notes Booklet: ✅ x1
Active Learning Game or Map: ✅ x1
BEST VALUE!
Topic Booklet: ✅ x1
Write Your Own Notes Booklet: ❌
Active Learning Game or Map: ❌
Topic Booklet: ❌
Write Your Own Notes Booklet: ✅ x1
Active Learning Game or Map: ❌
Please note, our resources are NOT to be photocopied. Thank you.
My daughter loves doing the workbook and playing the games. Would highly recommend.
Super books,take the work out of learning.
We haven't started using it as yet but we have had a good look and are excited to get going.
Extremely-well-designed and well-presented KS3 Science resource.
Pupils will benefit from clear, though detailed, factual presentation, broken down into manageable chunks.
Highly recommended.
The artwork and eye-friendly layout make KS3 science much less daunting. Student with dyslexia who had previously been quite fearful of science and shown little engagement used the OAKA topic guides to scaffold taught content and support classwork and has started to participate more in science lessons with greater confidence.
That's wonderful to hear, Oliver! Thank you so much for taking the time to let us know.
Chemistry
